Abstract
Background: FCR has been standard firstline treatment for young patients (pts) with CLL with a CR rate after 6 cycles of 40-72%, and undetectable bone marrow (BM) MRD rate after 6 cycles of 43-58%. However, there is a 5% risk of t-MDS/AML. Pts with mutated IGHV (IGHV -M) have favorable long-term outcomes (10-year PFS of >60%) after receiving first-line FCR with a plateau on the PFS curve. Ibrutinib, a BTK inhibitor, is approved for pts with CLL/SLL. Obinutuzumab, a glycoengineered type II CD20 mAb, was shown to be superior to rituximab in older adults when combined with chlorambucil in the CLL11 trial. Emerging data (HELIOS trial) indicated that the combination of ibrutinib with chemoimmunotherapy (CIT) is safe and effective. We developed a CIT-based regimen of finite duration that included ibrutinib and obinutuzumab. The intent was to limit chemotherapy to 3 courses, potentially reducing short- and long-term toxicity, while maintaining efficacy through the addition of ibrutinib and a more potent CD20 antibody (obinutuzumab) and achieving durable, therapy-free remission.
Methods: This is an investigator-initiated phase II trial with ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) for previously untreated pts with IGHV -M CLL (NCT02629809). Key eligibility included age ≥18, IGHV -M, no del(17p)/ TP53 mutation. Pts received 3 courses of iFCG. G-CSF was not mandated during the early part of the trial; however, due to increased neutropenia, the use of prophylactic G-CSF is now required with the iFCG courses. The primary endpoint is CR/CRi with undetectable BM MRD (4-color flow-cytometry, sensitivity 10-4) after 3 courses of iFCG. Pts meeting the primary endpoint received ibrutinib with obinutuzumab (iG) for C4-6, then ibrutinib C7-12. Pts not achieving the primary endpoint received iG (C4-12). All pts with undetectable MRD at 1 yr stop all therapy, including ibrutinib. Pts MRD+ at 1 year may continue ibrutinib. Response assessment is per IWCLL 2008 criteria with BM and CT scans every 3 months during first year, then Q6 months. The historical rate of C3 undetectable BM MRD with FCR in IGHV -M pts was 26% (Strati, Blood 2014). Target undetectable BM MRD after iFCG x3 is 45%; sample size is 45.
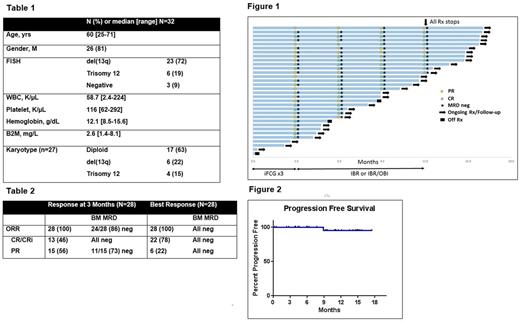
Results: Since April 2016, 32 pts initiated treatment. Median follow-up is 10.9 months (range, 0.2-17.5). Baseline characteristics are shown in Table 1. By trial design, all pts had IGHV -M. No pt had del(11q) or complex karyotype. Twenty-eight pts have completed 3 courses of iFCG and had an initial response assessment (of the remaining 4 pts, 3 have not yet completed 3 courses of treatment; 1 pt came off study after receiving day 1 of treatment with obinutuzumab and ibrutinib due to G3 infusion related reaction (IRR) and G4 thrombocytopenia). All 28 pts achieved a response; 24/28 (86%) achieved MRD-negative remission in the BM at 3 month (Table 2, Figure 1). Overall, 13/28 (46%) achieved CR/CRi with undetectable MRD at 3 months. All pts with PR had bulky adenopathy at baseline, and had residual lymphadenopathy ranging from 1.6 to 3.5 cm after 3 courses of iFCG. Responses continue to improve over time (6 months: CR/CRi rate 74%, MRD neg rate 91%; 12 months: CR/CRi rate 75%, MRD neg rate 100%). Twelve pts have reached the 12 month time-point (all 12 were MRD neg; 9 CR/CRi, 3 PR), and all 12 have stopped treatment per protocol design; all remain MRD negative at a median follow-up of 3.2 months (range, 0.3-4.4) after stopping therapy. Pretreatment B2M ≥4 mg/L was associated with a lower rate of undetectable BM MRD at 3 months (50% vs. 95%, p = 0.022). No patient has progressed, and all but 3 continue to receive treatment on protocol (Figure 2). Reasons for discontinuation included 1) death due to cardiac failure (see below), 2) pulmonary MAC infection, and 3) pt with IRR to obinutuzumab (described above).
G3-4 neutropenia occurred in 68% pts; G3-4 thrombocytopenia in 48% pts. Atrial fibrillation occurred in 2 pts. G3 ALT elevation occurred in 3 pts. FC was dose reduced in 55% pts; ibrutinib dose-reduced in 19% pts. There has been 1 death on the study. This was a 26 year old patient with no cardiac history who developed new onset congestive heart failure during cycle 9 of treatment with ibrutinib and obinutuzumab.
Conclusions: iFCG achieves high rate of undetectable MRD after 3 courses. All 12 pts who have reached the 1 year time point are MRD-negative and have stopped all therapy including ibrutinib. Enrollment, treatment and follow up continues.
Jain: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Abbvie: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Verastem: Research Funding; Genentech: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Thompson: Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Burger: Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Bose: Incyte Corporation: Honoraria. Takahashi: Symbio Pharmaceuticals: Consultancy. Kantarjian: Delta-Fly Pharma: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding. O'Brien: Astellas: Consultancy; Celgene: Consultancy; Sunesis: Consultancy; Vaniam Group LLC: Consultancy; Pfizer: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Aptose Biosciences, Inc.: Consultancy; GSK: Consultancy; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; AbbVie: Consultancy; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Acerta: Other: Research Support: Honorarium, Research Funding; Alexion: Consultancy; Janssen: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding; ProNAI: Other: Research Support: Honorarium, Research Funding. Wierda: Acerta: Research Funding; Merck: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Karyopharm: Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Kite: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Research Funding; Celgene: Consultancy, Honoraria; Juno: Research Funding; The University of Texas MD Anderson Cancer Center: Employment; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; Emergent: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal